Enoxaparin sodium
About Medicine
PDxane® is an antithrombotic agent, heparin group. PDxane® is a low molecular weight heparin with a mean molecular weight of approximately 4,500 daltons. The drug substance is the sodium salt.
Each 1mg Enoxaparin is equivalent to 100 IU anti-Xa activity. Enoxaparin sodium is characterized by a higher ratio of antithrombotic activity to anticoagulant activity than unfractionated heparin. At recommended doses, it does not significantly influence platelet aggregation, binding of fibrinogen to platelets or global blood clotting tests such as APTT and prothrombin time.
Prefilled syringes:
20 mg Enoxaparin sodium (equivalent to 2,000 IU anti-Xa activity) in 0.2 mL
40 mg Enoxaparin sodium (equivalent to 4,000 IU anti-Xa activity) in 0.4 mL
60 mg Enoxaparin sodium (equivalent to 6,000 IU anti-Xa activity) in 0.6 mL
80 mg Enoxaparin sodium (equivalent to 8,000 IU anti-Xa activity) in 0.8 mL
Sterile solution, clear and colorless in prefilled syringe
Overdosage
If you think that you have used too much or too little PDxane®, tell your doctor, nurse or pharmacist immediately, even if you have no signs of a problem.
What if you forget to use PDxane®?
If you forget to give yourself a dose, have it as soon as you remember. Do not give yourself a double dose on the same day to make up for a forgotten dose. Keeping a diary will help to make sure you do not miss a dose.
Effect on Ability to Drive and Operate Machinery
PDxane® has no effect on the ability to drive and operate machines.
The prophylaxis of thromboembolic disorders of venous origin, in particular those which may be associated with orthopedic or general surgery.
The prophylaxis of venous thromboembolism in medical patients bedridden due to acute illness.
The treatment of venous thromboembolic disease presenting with deep vein thrombosis, pulmonary embolism or both.
The treatment of unstable angina and non-Q-wave myocardial infarction, administered concurrently with aspirin.
The treatment of acute ST-segment elevation myocardial Infarction (STEMI) including patients to be managed medically or with subsequent Percutaneous Coronary Intervention (PCI) in conjunction with thrombolytic drugs (fibrin or non-fibrin specific).
The prevention of thrombus formation in the extracorporeal circulation during hemodialysis.
Prophylaxis of venous thromboembolism in medical patients
The recommended dose of PDxane® is 40 mg (4,000 IU) once daily by subcutaneous injection. Treatment with PDxane® is prescribed for a minimum of 6 days and continued until the return to full ambulation, for a maximum of 14 days.
Prophylaxis of venous thromboembolism
In patients with a low to moderate risk of venous thromboembolism the recommended dosage is 20 mg (2,000 IU) once daily by subcutaneous injection for 7 to 10 days, or until the risk of thromboembolism has diminished. In patients undergoing surgery, the initial dose should be given approximately 2 hours pre-operatively.
In patients with a higher risk, such as in orthopedic surgery, the dosage should be 40 mg (4,000 IU) daily by subcutaneous injection with the initial dose administered approximately 12 hours before surgery.
In patients with a high-risk of venous thromboembolism who undergo abdominal or pelvic surgery for cancer and are not otherwise at risk for major bleeding complications, the recommended dosage is 40 mg (4,000 IU) once daily by subcutaneous injection for 4 weeks with the initial dose administered approximately 12 hours before surgery.
Treatment of venous thromboembolism
PDxane® should be administered subcutaneously as a single daily injection of 1.5 mg/kg (150 IU/kg). PDxane® treatment is usually prescribed for at least 5 days and until adequate oral anticoagulation is established.
Treatment of unstable angina and non-Q-wave myocardial infarction
The recommended dose is 1 mg/kg PDxane® every 12 hours by subcutaneous injection, administered concurrently with oral aspirin (100 to 325mg once daily). Treatment with PDxane® in these patients should be prescribed for a minimum of 2 days and continued until clinical stabilization. The usual duration of treatment is 2 to 8 days.
Treatment of acute ST-segment elevation myocardial Infarction
The recommended dose of PDxane® is a single IV bolus of 30mg plus a 1mg/kg SC dose followed by 1mg/kg administered SC every 12 hours (max 100mg for the first two doses only, followed by 1mg/kg dosing for the remaining doses). For dosage in patients ≥75 years of age.
Prevention of extracorporeal thrombus formation during hemodialysis
A dose equivalent to 1 mg/kg (100 IU/kg) introduced into the arterial line at the beginning of a dialysis session is usually sufficient for a 4 hour session. If fibrin rings are found, such as after a longer than normal session, a further dose of 0.5 to 1mg/kg (50 to 100 IU/kg) may be given. For patients at a high risk of hemorrhage the dose should be reduced to 0.5 mg/kg (50 IU/kg) for double vascular access or 0.75 mg/kg (75 IU/kg) for single vascular access.
Not recommended, as dosage not established.
For treatment of acute ST-segment Elevation Myocardial Infarction in elderly patients ≥75 years of age, do not use an initial IV bolus. Initiate dosing with 0.75mg/kg SC every 12 hours (maximum 75mg for the first two doses only, followed by 0.75mg/kg dosing for the remaining doses).
For other indications, no dosage adjustments are necessary in the elderly, unless kidney function is impaired.
Severe renal impairment:
A dosage adjustment is required for patients with severe renal impairment (creatinine clearance < 30 mL/min), according to the following tables, since enoxaparin sodium exposure is significantly increased in this patient population:
Dosage adjustments for therapeutic dosage ranges
Standard dosing Severe renal impairment 1 mg/kg SC twice daily 1 mg/kg SC once daily 1.5 mg/kg SC once daily 1 mg/kg SC once daily For treatment of acute STEMI in patients <75 years of age 30mg-single IV bolus plus a 1mg/kg SC dose followed by 1mg/kg twice daily.(Max 100mg for each of the first two SC doses) 30mg-single IV bolus plus a 1mg/kg SC dose followed by 1mg/kg once daily.(Max 100mg for first SC dose only) For treatment of acute STEMI in elderly patients ≥75 years of age 0.75mg/kg SC twice daily without initial bolus.(Max 75mg for each of the first two SC doses) 1mg/kg SC once daily without initial bolus.(Max 100mg for first SC dose only) Dosage adjustments for prophylactic dosage ranges
Standard dosing Severe renal impairment 40 mg SC once daily 20 mg SC once daily 20 mg SC once daily 20 mg SC once daily The recommended dosage adjustments do not apply to the hemodialysis indication.
Moderate and mild renal impairment:
Although no dosage adjustments are recommended in patients with moderate renal impairment (creatinine clearance 30-50 mL/min) or mild renal impairment (creatinine clearance 50-80 mL/min), careful clinical monitoring is advised.
Hypersensitivity to either enoxaparin sodium, heparin or its derivatives including other Low Molecular Weight Heparins.
In patients with acute bacterial endocarditis
Active major bleeding and conditions with a high risk of uncontrolled hemorrhage, including recent hemorrhagic stroke.
Thrombocytopenia in patients with a positive in-vitro aggregation test in the presence of enoxaparin.
Active gastric or duodenal ulceration
In patients receiving heparin for treatment rather than prophylaxis, locoregional anesthesia in elective surgical procedures is contraindicated.
Spinal/epidural anesthesia
There have been cases of intra-spinal hematomas reported with the concurrent use of enoxaparin sodium and spinal/epidural anesthesia or spinal puncture resulting in long term or permanent paralysis. These events are rare with enoxaparin sodium dosage regimens 40 mg OD or lower. The risk is greater with higher enoxaparin sodium dosage regimens, use of post-operative indwelling catheters or the concomitant use of additional drugs affecting hemostasis such as NSAIDs. The risk also appears to be increased by traumatic or repeated neuraxial puncture or in patients with a history of spinal surgery or spinal deformity. To reduce the potential risk of bleeding associated with the concurrent use of enoxaparin sodium and epidural anesthesia/analgesia, the pharmacokinetic profile of the drug should be considered.
Enoxaparin is to be used with extreme caution in patients with a history of heparin-induced thrombocytopenia with or without thrombosis.
Enoxaparin injection, as with any other anticoagulant therapy, should be used with caution in conditions with increased potential for bleeding, such as: impaired hemostasis, history of peptic ulcer, recent ischemic stroke, uncontrolled severe arterial hypertension, diabetic retinopathy, recent neuro- or ophthalmologic surgery.
As with other anticoagulants, bleeding may occur at any site. If bleeding occurs, and sometimes anemia, the origin of the hemorrhage should be investigated and appropriate treatment instituted.
Heparin can suppress adrenal secretion of aldosterone leading to hyperkalemia, particularly in patients such as those with diabetes mellitus, chronic renal failure, pre-existing metabolic acidosis, a raised plasma potassium or taking potassium sparing drugs. The risk of hyperkalemia appears to increase with duration of therapy but is usually reversible. Plasma potassium should be measured in patients at risk before starting heparin therapy and monitored regularly thereafter particularly if treatment is prolonged beyond about 7 days.
Prosthetic Heart Valves
In low-weight women (< 45 kg) and low-weight men (< 57 kg), an increase in enoxaparin exposure has been observed within the prophylactic dosage ranges (non-weight adjusted), which may lead to a higher risk of bleeding.
Low Molecular Weight Heparins should not be used interchangeably since they differ in their manufacturing process, molecular weights, specific anti Xa activities, units and dosage.
Pregnancy Risk Factor is B. Adverse events were not observed in animal reproduction studies. Low molecular weight heparin (LMWH) does not cross the placenta; increased risks of fetal bleeding or teratogenic effects have not been reported.
Limited information indicates that maternal enoxaparin in doses up to 40 mg daily do not to cause any adverse effects in breastfed infants. Because it’s large molecular weight of 2000 to 8000 Daltons, enoxaparin would not be expected to be excreted into breast milk or to be absorbed from breast milk by the infant. No special precautions are required.
As with all anticoagulants, bleeding is the major adverse effect of enoxaparin. Hemorrhage may occur at virtually any site. Risk is dependent on multiple variables. At the recommended doses, single injections of enoxaparin do not significantly influence platelet aggregation or affect global clotting time (i.e. PT or aPTT).
Common (1-10%):
Allergic reaction, Thrombocytopenia, Hepatic enzymes increase (mainly transaminases), Urticaria, Pruritus, Erythema, Injection site hematoma, Injection site pain, Other injection site reaction, Diarrhea, Nausea, Fever, Confusion, Hematuria, Headache.
Uncommon (0.1-1%):
Intracranial hemorrhage, Retroperitoneal hemorrhage, Bullous dermatitis, Local irritation, Skin necrosis at injection site, Hypertriglyceridemia, Osteoporosis following long-term therapy (greater than 3 months).
Rare (<0.1%):
Hyperkalemia, Retroperitoneal Hemorrhage (in patients with unstable angina and non-Q-wave MI and Prophylaxis in surgical patients), Anaphylactic reaction, Anemia
It is recommended that agents which affect hemostasis should be discontinued prior to enoxaparin therapy unless their use is essential.
Other anticoagulant and antiplatelet drugs: Heparin, Warfarin, Dipyridamole, Ticlopidine and Clopidogrel
Dabigatran, Rivaroxaban, Apixaban
NSAIDs: Ibuprofen, Ketorolac, Diclofenac and other drugs in this category
Dextran
SSRIs: Fluoxetine
Thrombolytics agents: May enhance the anticoagulant effect of Anticoagulants. Management: see full drug monograph for guidelines for the use of Alteplase for acute ischemic stroke during treatment with oral anticoagulants.
Aspirin and other salicylate: may enhance the anticoagulant effect of Anticoagulants.
Dasatinib
Pentoxifylline: may enhance the anticoagulant effect of Heparin (low molecular weight).
Deferasirox
Potassium-sparing diuretics: Heparin (low molecular weight) may enhance the hyperkalemic effect of potassium-sparing diuretics. Management: monitor serum potassium concentrations closely.
ACEIs: Captopril, Enalapril
Aliskiren
Potassium salts: Heparin (low molecular weight) may enhance the hyperkalemic effect of Potassium salts.
Estrogen derivatives: may diminish the anticoagulant effect of Anticoagulants.
Omega3 Fatty Acids: may enhance the anticoagulant effect of Anticoagulants.
Vitamin E: may enhance the anticoagulant effect of Anticoagulants. Vitamin E may also increase the overall risk for bleeding
Avoid cat’s claw, dong quai, evening primrose, feverfew, garlic, ginger, ginkgo, red clover, horse chestnut, green tea, ginseng (all have additional antiplatelet activity).
Complete blood count; platelets, hematocrit, hemoglobin, occult blood, anti-Xa levels, serum creatinine; monitoring of PT and/or aPTT is not necessary. Routine monitoring of anti-Xa levels is not required, but has been utilized in patients with obesity and/or renal insufficiency. Monitoring anti-Xa levels is recommended in pregnant women receiving therapeutic doses of enoxaparin or when receiving enoxaparin for the prevention of thromboembolism with mechanical heart valves. For patients >190 kg, if anti-Xa monitoring is available, adjusting dose based on anti-Xa levels is recommended; if anti-Xa monitoring is unavailable, reduce dose if bleeding occurs.
PDxane® binds to anti-thrombin III leading to inhibition of coagulation factors IIa and Xa.
Onset of action: Peak effect: SubQ: Anti-Xa and antithrombin (anti-IIa): 3-5 hours
Metabolism: Hepatic, to lower molecular weight fragments (little activity)
Protein binding: Does not bind to heparin binding proteins
Distribution:3 L (based on anti-Xa activity)
Half-life elimination, plasma: 2-4 times longer than standard heparin, independent of dose; based on anti-Xa activity: 4.5-7 hours
Excretion: Urine (40% of dose; 10% as active fragments)
Wash your hands and the area that you will inject with soap and water then dry them.
Sit or lie in a comfortable position so you are relaxed.
Make sure you can see the place you are going to inject. A lounge chair, recliner, or bed propped up with pillows is ideal.
Choose an area on the right or left side of your stomach. This should be at least 5 centimeters away from your belly button and out towards your sides.
Remember: Do not inject yourself within 5 centimeters of your belly button or around existing scars or bruises.
Change the place where you inject between the left and right sides of your stomach, depending on the area you were last injected.
Carefully pull off the needle cap from the PDxane® The syringe is pre-filled and ready to use.
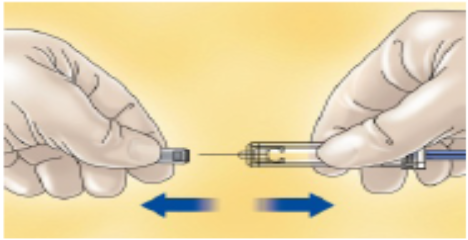
Do not throw away the needle cap.
Once you have removed the cap, do not allow the needle to touch anything. This is to make sure the needle stays clean (sterile).
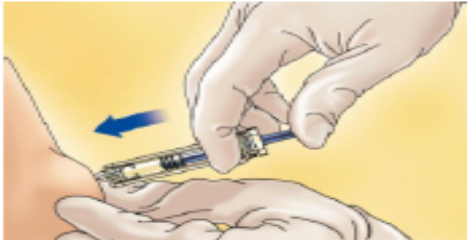
Hold the syringe in the hand you write with (like a pencil) and with your other hand, gently pinch the cleaned area of your abdomen between your forefinger and thumb to make a fold in the skin.
Make sure you hold the skin fold throughout the injection.
Hold the syringe so that the needle is pointing downwards (vertically at a 90° angle).
Insert the full length of the needle into the skin fold.
Press down on the plunger with your finger. This will send the medication into the fatty tissue of the stomach.
Make sure you hold the skin fold throughout the injection.
Put the needle cap back on the needle.
You can now let go of the skin fold.
To avoid bruising, do not rub the injection site after you have injected yourself.
PDxane® should not be mixed with any other injections or infusions.
Do not store above 25°.
Keep the container in the outer carton in order to protect from light.
Keep out of the reach and sight of children.
Do not use PDxane® after the expiry date which is stated on the box and on the syringe label (EXP). The expiry date refers to the last day of that month.
Do not freeze PDxane®.
Do not shake PDxane®.
PDxane® pre-filled syringes are single dose containers, discard any unused product.
Water for injection